Pressure and gravity
Question:
Gravity causes pressure to increase with depth in the ocean and atmosphere. If gravity only points downward, how can pressure act in any direction, and how can this be understood at the particle level? How can ocean pressure increase with depth if water is incompressible?
Answer:
This question addresses many different concepts in physics, ranging from microscopic to macroscopic interactions. Understanding physical interaction at multiple scales can be difficult.
First let's consider a balloon. The air molecules in the balloon move randomly, so there are collisions in all directions---this is why pressure operates in all directions. No matter which sides of the balloon you squeeze, the balloon will push back on your hand the same amount.
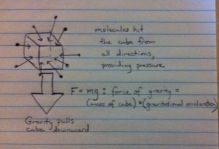
Now let's imagine a cube of air floating in the atmosphere. Here's a diagram showing the forces on the cube:
If the cube is floating in place---not falling down or going up---there must be zero net force on the cube. That means the forces in every direction must balance out. Since the cube is in a 3-dimensional atmosphere, there are three directions in which the forces need to balance: left-right, up-down and forward-backward. We can therefore write three equations:
Force upward = Force downward Force frontward = Force backward Force on left = Force on right
Left-right and forward-backward are easy. If temperature and air density don't change left-right or forward-backward, the forces from all the molecules hitting the cube on the left, right, front and back balance out, so there is no net force on the cube. In Earth's atmosphere, the temperature and density change vertically much faster than they change left-right or forward-backward: think of the subzero temperatures outside an airplane at 35,000 feet (7 miles). Now imagine traveling seven miles to a nearby town. Chances are the weather is very similar to your hometown.
So, why do the temperature and density change so dramatically in the vertical direction? It's because of gravity. We have both an upward and a downward force on the cube. The downward forces come from molecules hitting the top of the cube---pressure---and gravity. The upward force comes only from molecules hitting the bottom of the cube: upward pressure. The only way for the forces to equalize is for the upward pressure to be higher than the downward pressure. This configuration is called hydrostatic equilibrium: pressure increases from top to bottom so that there is no net force on any cube of air.
With a compressible fluid like air, it's easy to imagine how this pressure balance works: the fluid will get denser as you go downward, so more molecules will collide with the underside than the top of the cube. But what about an incompressible fluid like water? Water isn't 100% incompressible, but it's darn close: between sea level and the average ocean depth of 4,267 meters, the ocean density changes from 1.025 grams per cubic centimeter to 1.028 grams per cubic centimeter. That's only a 0.3% increase. However, between sea level and the average ocean depth, the pressure changes by a factor of 370. Clearly, the density change can't explain such a huge pressure difference.
While pressure in air is due to the motion of molecules, pressure in liquid water is different. It's easy to compress air because the molecules are very far apart, but in liquid water the molecules are as close as they can get. Water pressure comes from electrons in neighboring molecules repelling each other. However hard gravity pulls on ocean water, the water molecules push back just as hard. Pushing back against the weight of the ocean's upper layers, water in the ocean's deep trenches can reach pressures of over 1,000 atmospheres.
-Colby Haggerty and Sally Dodson-Robinson